How to teach covalent bonding | CPD
A chemical bond holds the parts of a chemical structure together. When the bond is between two non-metallic atoms, a covalent bond is formed.
Covalently bound species can fall into one of two categories:
- Elements in which all atoms are of the same nonmetal
- Compounds in which two or more different nonmetal atoms are bonded together.
Different models are used in 14–16 to represent covalently bound species (see Figure 1 for methane).
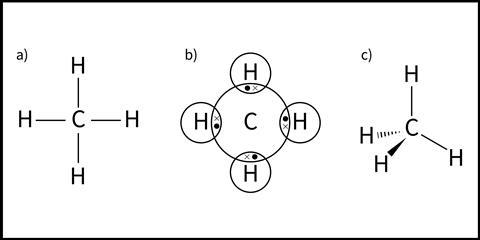
In the simplest model (Figure 1a), a covalent bond is represented by a straight line between atoms identified by their element symbols. In dot-cross diagrams, commonly introduced at 14–16 (Figure 1b), the outer shell electrons of each atom are shown in circular orbits. Covalent bonds are represented by a shared pair of electrons in an area of overlap.
None of these models show the 3D shape of the molecule. Switching between the 2D representation and the 3D form of a molecular model can be a conceptual challenge for students. A third model (Figure 1c) helps to overcome this difficulty. Dashes and wedges represent ties going in and out of the page, respectively.
what you need to know
With 14–16, students typically describe a covalent bond as sharing a pair of electrons. They can further explain that the positively charged nuclei of the bonded atoms are attracted to the shared pair of electrons by electrostatic forces, making covalent bonds very strong. But what about the repulsion between the electrons?
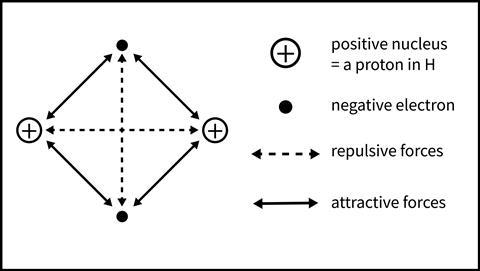
Students will better understand bonding in a simple covalent molecule by considering all of the electrostatic interactions involved. Figure 2 shows the interactions between the two nuclei and electrons in the diatomic molecule H2. Within the molecule there are both attractive (proton-electron) and repulsive (electron-electron and proton-proton) forces. The strength of these forces varies with the reciprocal of the square of the distance between the particles involved. Therefore, as the atoms approach each other, the strength of the attractive and repulsive forces changes. At a certain distance, the equilibrium distance between the nuclei, the net attractive interaction is maximized. The molecule is most stable at this distance between the atoms.
The model described above is limited because it represents the protons and electrons as point charges. To better understand covalent bonding, it is necessary to look at a more sophisticated model of atomic structure. After 16, electrons are no longer considered as point particles, but as a cloud of negative charge. An electron fills a volume in space called an atomic orbital. Density diagrams give the probability of finding an electron in a given space. The orbital diagrams known from FIG. 16 are a simplification of density diagrams. A single line is drawn to represent the outer edge of an area where there is a 90% chance of finding the electron. The shapes of the orbitals and their overlap determine the strength and type of covalent bonds formed.
clear up misunderstandings
Dot-cross diagrams and the idea of ”sharing” electrons can lead students to believe that electrons “belong” to a particular atom. Students can think of shared pairs of electrons as the “glue” that “joins” atoms together. Simple molecular modeling kits can embed this misconception—covalent bonds are represented as physical objects holding spherical atoms together.
What the model lacks
Ideas that cannot be explained using the 14-16 model of covalent bonding include:
- the difference between a single and a double covalent bond
- the reactivity of alkenes
- Delocalization of electrons in conjugated systems
- Dipole moments and polar bonds.
It is important to emphasize the electrostatic nature of the attractions that hold the two atoms together in a covalent bond. Quite early in secondary school, students are familiar with the idea that opposite charges attract and like charges repel. Once students have learned about scatter and cross plots, I’ll start the next lesson with this activity, which is taken from the Covalent Bonds Classroom Resources. I ask you to annotate the diagram of a hydrogen molecule (Figure 3) to show all the attractive and repulsive forces that would exist. This simple activity encourages students to think beyond the idea of shared electrons for covalent bonds.
The idea that matter is held together by electrostatic forces of attraction is described by Jasper Green as one of 13 powerful ideas – the ideas are the most conceptually important pieces of science that we want to teach students in school. This shift in focus to electrostatics can be applied to all types of bonding 14–16. Students will gain a better understanding of the connections between different types of attachment. They will be better prepared to move on to more complex ideas about attachments that they will encounter after 16.
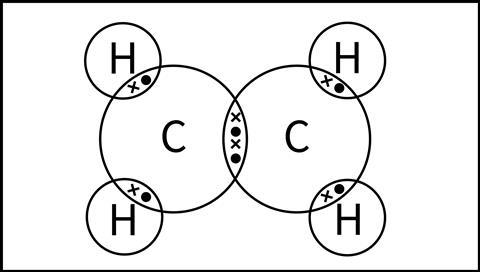
At this stage, we would draw an ethene molecule as a dot-cross diagram as shown in Figure 4. Although this model shows the connectivity and explains the existence of a double bond between the two carbon atoms, it does not explain the molecular shape or its reactivity.
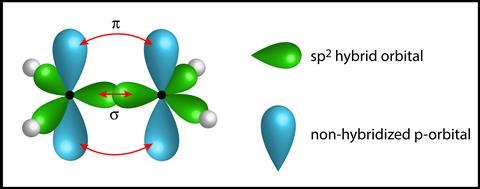
To fully understand the distribution of electrons in ethene, students must understand the process of hybridization, which goes well beyond the 14-16 curriculum. However, the concept of orbital overlap can be introduced. Each carbon atom has four electrons to share. Three are held in identical orbitals (sp2), spaced as far apart as possible to minimize electrostatic repulsion, and the fourth is kept within an orbital. A simple schematic representation (like Figure 5) allows students to see the shape of the ethene molecule and understand its reactivity due to the high energy electrons held in the weaker pi bond resulting from the “sideways” overlap of two parallel p -orbitals is formed.
Take Home Points
- When you teach bonds, you shift the focus away from atoms, which must be given a complete outer shell; Instead, focus on the electrostatic nature of chemical bonding.
- Consider teaching metallic and ionic bonding first (since the electrostatic interactions are more obvious) before moving on to covalent bonding.
- Encourage students to think about electrons that exist in clouds around a molecule by showing students space-filled models for molecules as well as the simpler ball and stick models. Try MolView.
- As you introduce the different models of covalent bonding, make it clear what their strengths and weaknesses are.
- Encourage interested students to continue reading about Lennard Jones’ potential for modeling particle-particle interactions.