Latest in lab automation
In this article Drug Target Review’s Izzy Wood highlights three of the latest discoveries using lab automation techniques and technologies that are helping scientists.
Laboratory automation is the integration of automated technologies into the laboratory to enable new and improved processes1.
Typically, lab automation provides solutions that address repetitive tasks in a lab, such as: B. Eliminate the handling of liquids normally performed by scientists1.
As technology advances, more complex processes, even entire workflows, can now be automated. Automated technology is increasingly a requirement in today’s labs to remain competitive and meet demand.
In this article, Drug Target Review’s Izzy Wood highlights the latest discoveries in laboratory automation.
Database could predict cancer
Cancer predictive medicine has received a boost with the recent unveiling of a new cancer protein profile database compiled from artificial intelligence (AI) and machine learning. The new open-access Disease Blood Atlas provides a first-ever map of the proteomic signature in the blood of cancer patients.
The new database, created by the Human Protein Atlas consortium,2 based at SciLifeLab, a joint research center that includes KTH Royal Institute of Technology, Uppsala University, Karolinska Institutet and Stockholm University, all Sweden, highlights 1,463 proteins associated with 12 different types of cancer, and introduces proteins that can be used to identify individual cancer types based on a drop of blood.
The Disease Blood Atlas was compiled from microscopic measurements of blood plasma collected from 1,400 cancer patients at the time of diagnosis and before treatment. The blood samples were subjected to a combination of statistical analysis of gene expression and machine learning-based disease prediction.
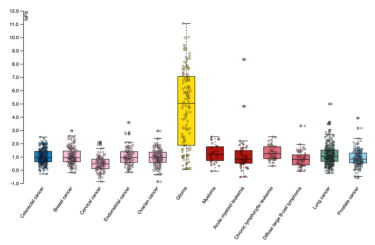
The yellow bar in this reading of the protein GFAP indicates increased expression in the blood of patients with brain tumors [Credit: Human Disease Blood Atlas]
The section called “Protein 3-D Structure” shows the 3-dimensional structures for all human proteins using an AI-based prediction model (AlfaFold). In addition, a major update to the Tissue Beatlas section provides detailed spatial multiplex profiles of proteins specific to human testes and kidneys. Additional data for single cell analysis of tissues and organs as well as data from an extensive catalog of human cell lines are also provided.
“We believe that the new sections of the open-access Human Protein Atlas, with large amounts of novel data covering all human proteins, will provide new dimensions of valuable information for researchers interested in human biology and disease,” Uhlén concluded .
You can read the whole article here.
Mathematical model predicts long-term effectiveness of COVID-19 vaccine
Researchers at Massachusetts General Hospital (MGH), USA, recently developed a mathematical model that can predict the course of vaccine-induced immunity against COVID-19 in different patient populations, including otherwise healthy individuals and those with cancer or suppressed immune responses.
The model contains different variants of SARS-CoV-2.
The model also makes predictions under possible future scenarios (such as the emergence of SARS-CoV-2 variants with greater immune evasion) and shows the advantages of the new bivalent vaccines.
The model published in PNASbuilds on the mathematical framework the researchers previously developed, which they used to understand why treatment responses vary widely in people with COVID-19 and to identify biological markers associated with these varied responses.
In this latest work, also published in PNAS,2 Scientists addressed the need to predict vaccine effectiveness over time.
“We used this model to simulate how differences in virus, patient, and vaccine characteristics may affect COVID-19 outcomes,” said senior author Dr. Rakesh K. Jain, director of the EL Steele Laboratories for Tumor Biology at MGH.
The model accounts for different variants of SARS-CoV-2 (including hypothetical ones), original and bivalent forms of the vaccine, and different considerations for specific patients, such as: B. Interactions between the virus, immune cells and tumor cells in individuals with cancer.
The model predicted that a booster dose of either Pfizer-BioNTech or Moderna mRNA vaccines can induce greatly enhanced antibody and immune cell-based responses against SARS-CoV-2 to provide adequate protection for more than a year in healthy individuals offer.
“Our results could help determine the timing of booster vaccinations in individuals with different characteristics and comorbidities, as well as for novel virus variants,” Jain added.
Read more about the study here.
Computer model determines drug candidate’s ability to bind to proteins
By combining computational physics with experimental data, researchers at the University of Arkansas, USA, have developed computer models to determine a drug candidate’s ability to target and bind to proteins in cells. The work was recently published in natural informatics.3
If such an estimator is accurate, it could prove the binding affinity computationally, thereby discouraging experimental researchers from studying millions of chemical compounds. The work could significantly reduce the costs and time involved in developing new drugs.
“We have developed a theoretical framework to estimate ligand-protein binding,” said Associate Professor Mahmoud Moradi. “The proposed method assigns an effective energy to the ligand at each lattice point in a coordinate system that originates at the most likely location of the ligand in the bound state.”
Moradi and Thallapuranam used biased simulations—as well as nonparametric rebalancing techniques to account for bias—to produce an authoritative estimator that was computationally efficient and accurate. They then used a mathematically robust technique called orientational quaternion formalism to further describe the conformational changes of the ligand upon binding to target proteins.
The researchers tested this approach by estimating the binding affinity between human fibroblast growth factor 1 – a specific signaling protein – and heparin hexasaccharide 5, a popular drug.
The project was conceived because Moradi and Thallapuranam were studying human fibroblast growth factor-1 protein and its mutants in the absence and presence of heparin. They found strong qualitative agreement between simulations and experimental results.
“In terms of binding affinity, we knew that the typical methods we had at our disposal wouldn’t work on a problem this difficult,” Moradi said. “That’s why we decided to develop a new method. We had a joyous moment when the experimental and calculated data were compared and the two numbers matched almost perfectly.”
Learn more here.
References:
- What is lab automation? [Internet]. vending machines. 2023 [cited 2023Feb9]. Available at: https://automata.tech/what-is-lab-automation/
- The Human Protein Atlas [Internet]. The Human Protein Atlas. [cited 2023Feb9]. Available at: https://www.proteinatlas.org/
- Voouturi C, Hardin CC, Naranbhai V, Nikmaneshi MR, Khandekar MJ, Gainor JF, et al. Mechanistic model for the efficacy of booster doses in healthy, cancer and immunocompromised patients infected with SARS-COV-2. Proceedings of the National Academy of Sciences. 2023;120(3).
- Govind Kumar V, Polasa A, Agrawal S, Kumar TK, Moradi M. Binding affinity estimation from constrained umbrella sampling simulations. natural informatics. 2022;3(1):59-70.
Related topics
Analysis, Analysis Techniques, Artificial Intelligence, Big Data, Bioinformatics, Covid-19, Drug Development, Drug Research Processes, Informatics, Laboratory Automation, Protein, Protein Expression, Targets